- Home
- Adpkd Autosomal Dominant Polycystic Kidney Disease Treatment Market

ADPKD (Autosomal dominant polycystic kidney disease) Treatment Market by Type of Treatment (Pharmacological treatment and Surgical treatment), by End-User (Hospitals, Clinics, and Ambulatory Surgical Centres): Global Opportunity Analysis and Industry Forecast, 2022-2031
- Published Date: April, 2023 | Report ID: CLS-1922 | No of pages: 250 | Format:

The ADPKD (Autosomal dominant polycystic kidney disease) Treatment market was valued at $XX million in 2022. It is projected to grow at a CAGR of 5.9% from 2023 to 2031 and reach more than XX million by the end of 2031.
Analysts’ Viewpoint
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic disorder that causes multiple cysts to grow in the kidneys, leading to kidney failure in many patients. The current treatment options for ADPKD include managing the symptoms and complications associated with the disease, such as hypertension, pain, and infection, and slowing the progression of the disease.
There are currently two drugs approved by the FDA for the treatment of ADPKD: tolvaptan and everolimus. Tolvaptan works by blocking a hormone called vasopressin, which stimulates cyst growth in the kidneys. Everolimus is an immunosuppressant drug that slows down cell division and cyst growth. Both drugs have shown some effectiveness in slowing the progression of the disease in clinical trials.
The market for ADPKD treatments is expected to grow in the coming years, as the prevalence of the disease increases due to aging populations and improved diagnostic capabilities. However, the high cost of these drugs may limit their widespread use, and more research is needed to develop effective and affordable treatments for this devastating disease.
ADPKD (Autosomal dominant polycystic kidney disease) Treatment Overview
ADPKD (Autosomal Dominant Polycystic Kidney Disease) is a genetic disorder that affects the kidneys. It is caused by mutations in the genes PKD1 or PKD2, which encode for proteins that are involved in the development and maintenance of kidney cells. These mutations cause the formation of multiple fluid-filled cysts in the kidneys, which can grow over time and eventually lead to kidney failure.
ADPKD is an autosomal dominant disorder, which means that a person only needs to inherit one copy of the mutated gene from one parent to develop the disease. The other copy of the gene is normal, but it can still be passed on to future generations. The symptoms of ADPKD can vary from person to person, but they typically include pain in the back or sides, frequent urinary tract infections, blood in the urine, high blood pressure, and kidney stones. The disease can also cause complications such as liver cysts, aneurysms, and heart valve abnormalities.
ADPKD is a relatively common genetic disorder, affecting approximately 1 in 400 to 1 in 1,000 individuals worldwide. The disease is the most common inherited kidney disease and is responsible for up to 10% of all cases of end-stage renal disease (ESRD) requiring dialysis or kidney transplantation. ADPKD can present at any age, but symptoms usually appear in midlife, and the disease is more prevalent in adults than in children.
The limited treatment options for ADPKD create a significant unmet medical need for patients with the disease, driving the development of new therapies. Researchers are exploring new therapies that target the underlying mechanisms of the disease, such as drugs that inhibit cyst growth. Additionally, gene therapy and stem cell-based therapies are being studied as potential treatments for ADPKD. The high prevalence of ADPKD, coupled with the lack of a cure and limited treatment options, creates a significant economic burden on healthcare systems and patients. The cost of treating ADPKD patients with ESRD is estimated to be between $50,000 and $100,000 per year, per patient. Additionally, the indirect costs of the disease, such as lost productivity and reduced quality of life, are significant.
Moreover, governments, academic institutions, and pharmaceutical companies are investing heavily in R&D to develop new treatments for ADPKD. The high prevalence of the disease and its significant economic burden make it an attractive target for investment. In recent years, there has been a significant increase in the number of clinical trials investigating new treatments for ADPKD, reflecting the growing interest in this area. Two drugs have been approved by the FDA for the treatment of ADPKD: tolvaptan and everolimus. Tolvaptan works by blocking a hormone called vasopressin, which stimulates cyst growth in the kidneys. Everolimus is an immunosuppressant drug that slows down cell division and cyst growth. Both drugs have been shown to slow the progression of ADPKD in clinical trials, but their high cost limits their widespread use.
Despite the approval of these drugs, there is still a significant unmet medical need for ADPKD patients, and there is a growing demand for the development of new and more effective therapies. Researchers are exploring new therapies that target the underlying mechanisms of the disease, such as drugs that inhibit cyst growth. Gene therapy and stem cell-based therapies are also being studied as potential treatments for ADPKD.
The increasing investment in R&D for ADPKD is expected to lead to the development of new treatments that are more effective and have fewer side effects than the currently available drugs. These treatments have the potential to improve the lives of ADPKD patients and reduce the economic burden of the disease on healthcare systems and patients.
New product launches to flourish in the market
Pharmaceutical companies are investing heavily in research and development to develop effective therapies for ADPKD. In August 2020, Sanofi and Regeneron Pharmaceuticals announced that they were investing $2 billion over five years in the development of new therapies for various diseases, including ADPKD. In July 2020, Estela’s Pharma Inc. and FibroGen Inc. announced a strategic collaboration to develop and commercialize roxadustat, a novel oral drug for the treatment of anaemia in patients with chronic kidney disease, including ADPKD.
Segment Overview:
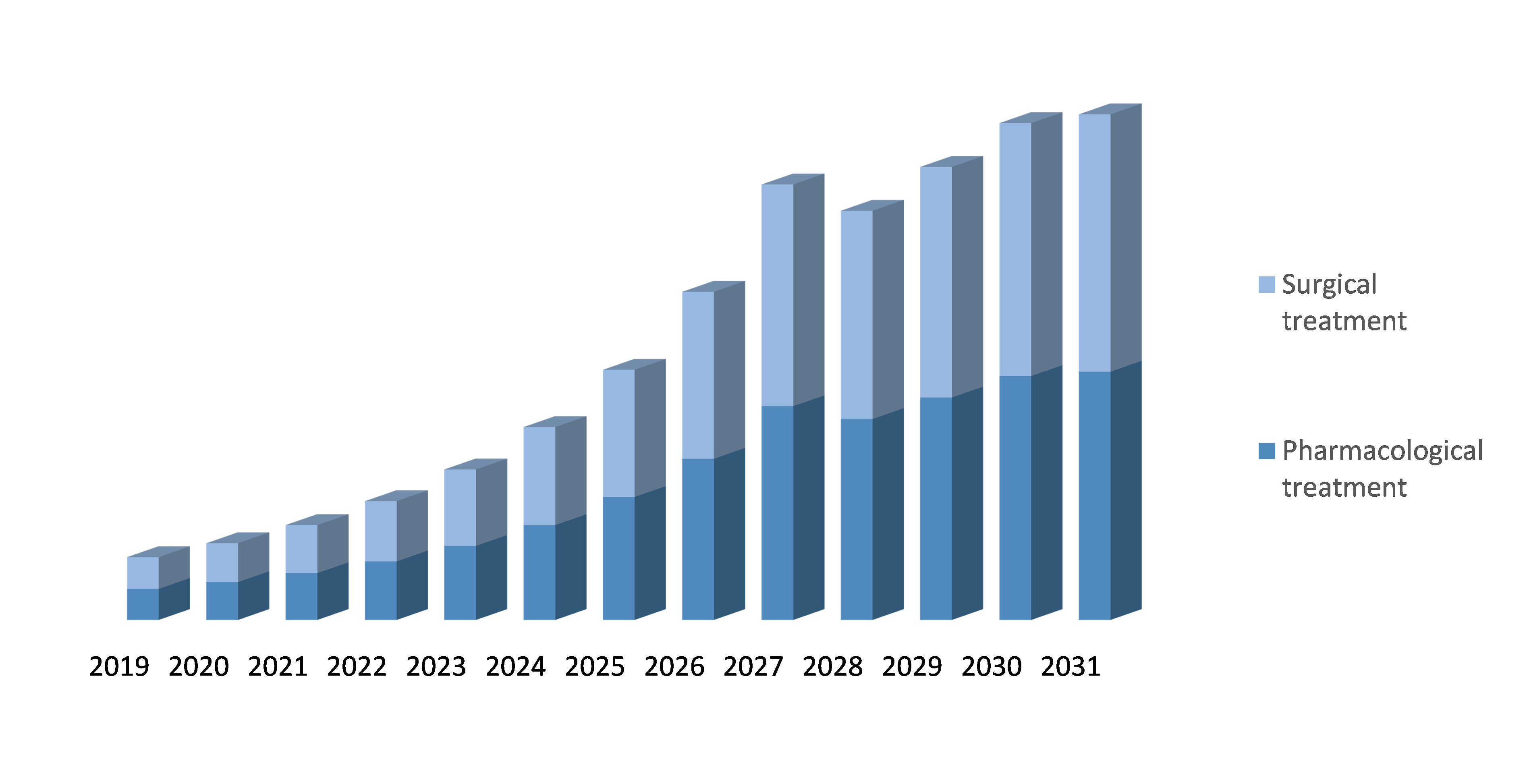
By Type of Treatment: The ADPKD (Autosomal dominant polycystic kidney disease) Treatment market is divided into Pharmacological treatment and Surgical treatment. Pharmacological treatment involves the use of drugs to slow down the growth and progression of cysts in the kidneys. This includes drugs such as tolvaptan, which has been approved by the FDA for the treatment of ADPKD, and everolimus, which is currently in clinical trials for the treatment of ADPKD. Surgical treatment involves the removal of the affected kidney or kidneys, typically in cases where the cysts are causing significant damage to the kidney and other organs. This may include procedures such as nephrectomy or kidney transplant. The choice of treatment depends on various factors, including the severity of the disease, the age and overall health of the patient, and the presence of any other medical conditions.
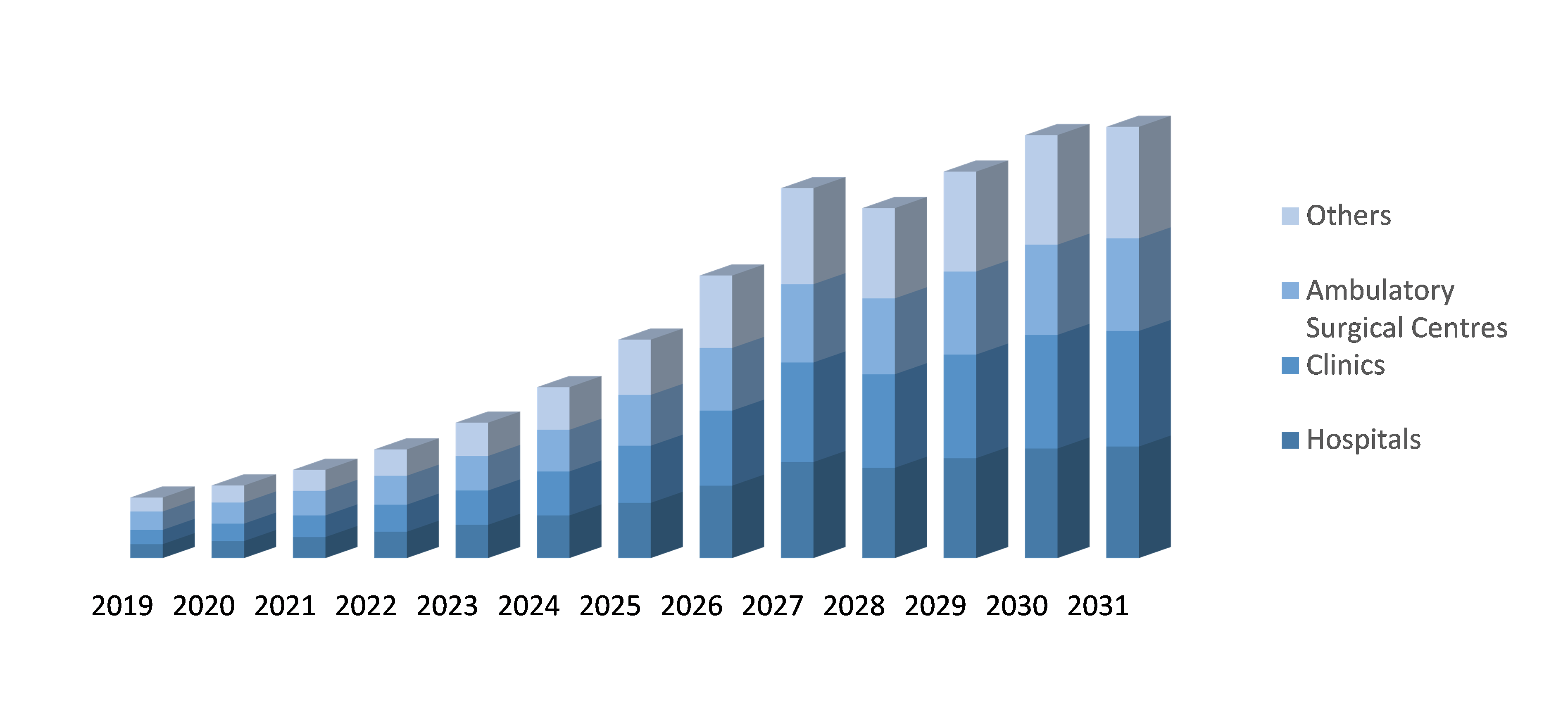
By End-user: The ADPKD (Autosomal dominant polycystic kidney disease) Treatment market is segmented by age, with Hospitals, Clinics and Ambulatory Surgical Centers. Hospitals are the most common healthcare facilities where ADPKD treatment is provided. Hospitals provide a wide range of medical and surgical services, including advanced imaging studies, drug therapies, and surgical interventions such as nephrectomy or kidney transplant. Clinics are smaller healthcare facilities that may specialize in the treatment of specific medical conditions. ADPKD clinics may offer specialized services such as genetic testing, counseling, and disease management programs, in addition to drug therapies and other treatments. Ambulatory surgical centers are outpatient facilities that provide surgical services for a wide range of medical conditions, including ADPKD. These facilities may offer minimally invasive procedures such as laparoscopic cyst removal, which may be appropriate for certain patients with ADPKD.
By Region:
The North American ADPKD (Autosomal dominant polycystic kidney disease) treatment market is one of the largest and most developed in the world. This is due to the high prevalence of ADPKD in the region, as well as the well-established healthcare infrastructure and high healthcare expenditure in countries such as the United States and Canada. The availability of advanced medical technologies, a large number of well-equipped hospitals and clinics, and high patient awareness about ADPKD are some of the key factors driving the growth of the North American market. Additionally, the presence of major pharmaceutical companies and ongoing research and development activities are also contributing to the growth of the market.
The Asia Pacific (APAC) ADPKD treatment market is experiencing significant growth due to factors such as the increasing prevalence of ADPKD in the region, the rising awareness about the disease, and increasing investment in healthcare infrastructure. Additionally, the availability of low-cost generic drugs and increasing government initiatives to provide affordable healthcare are also contributing to the growth of the market. The growing number of clinical trials and increasing research and development activities by pharmaceutical companies in the region are expected to further drive the market growth in the coming years.

Competitive analysis and profiles of the major players in the ADPKD (Autosomal dominant polycystic kidney disease) Treatment market, such as Biocodex S.A., Eisai Co., Ltd., GW Pharmaceuticals plc., H. Lundbeck A/S, Neurocrine Biosciences, Inc., Novartis AG, Pfizer Inc., Sanofi S.A., Takeda Pharmaceutical Company Limited and UCB S.A. Major players have adopted product launch and acquisition as key developmental strategies to improve the product portfolio of the ADPKD (Autosomal dominant polycystic kidney disease) Treatment market.
Market Scope and Structure Analysis
|
Report Metric |
Details |
|
Market Size Available for Years |
2021–2031 |
|
Base Year Considered |
2022 |
|
Forecast Period |
2023–2031 |
|
Forecast Unit |
Value (USD) |
|
Segments Covered |
By Type of Treatment, and End-user, and Region |
|
Regions Covered |
North America, Europe, Asia-Pacific, LAMEA |
|
Companies Covered |
|
Key Segments Covered
Type of Treatment
- Pharmacological treatment
- Vasopressin V2 receptor antagonist
- Angiotensin converting enzyme inhibitors
- Angiotensin receptor blockers
- mTOR inhibitors
- Somatostatin analogs
- Surgical treatment
End-user
- Hospitals
- Clinics
- Ambulatory Surgical Centres
Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Rest of Europe
- Asia-Pacific
- China
- Japan
- South Korea
- India
- Australia
- Rest of Asia-Pacific
- LAMEA
- Latin America
- Middle East
- Africa
TABLE OF CONTENT
- Research Methodology
- Desk Research
- Real-time insights and validation
- Forecast model
- Assumptions and forecast parameters
- Assumptions
- Forecast parameters
- Data sources
- Primary
- Secondary
- Executive Summary
- 360° summary
- By Type of Treatment
- By Disease Stage trends
- By End-User trends
- Market Overview
- Market segmentation & definitions
- Key takeaways
- Top investment pockets
- Top winning strategies
- Porter’s five forces analysis
- Bargaining power of consumers
- Bargaining power of suppliers
- Threat of new entrants
- Threat of substitutes
- Competitive rivalry in the market
- Market dynamics
- Drivers
- Restraints
- Opportunities
- Technology landscape
- Regulatory landscape
- Patent landscape
- Market value chain analysis
- Strategic overview
- ADPKD (Autosomal dominant polycystic kidney disease) Market, by Type of Treatment
- Pharmacological Type of Treatment
-
- Vasopressin V2 receptor antagonist
- Angiotensin converting enzyme inhibitors
- Angiotensin receptor blockers
- mTOR inhibitors
- Somatostatin analogs
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
-
- Surgical Type of Treatment
-
- Nephrectomy
- Cyst removal
- Kidney transplantation
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
-
- Pharmacological Type of Treatment
- ADPKD (Autosomal dominant polycystic kidney disease) Market, by Disease Stage
- Early Stage
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Moderate Stage
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Advanced Stage
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Early Stage
- ADPKD (Autosomal dominant polycystic kidney disease) Market, by End-User
- Hospitals
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Clinics
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Ambulatory Surgical Centers
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Hospitals
- ADPKD (Autosomal dominant polycystic kidney disease) Market, by Region
- North America
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User, 2022-2031
- Market size and forecast, by country, 2022-2031
- Comparative market share analysis, 2022 & 2031
- U.S.
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- Canada
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- Mexico
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- Europe
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Market size and forecast, by country, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Germany
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- UK
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- France
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- Spain
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- Italy
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- North America
-
-
- Rest of Europe
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- Rest of Europe
- Asia Pacific
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Market size and forecast, by country, 2022-2031
- Comparative market share analysis, 2022 & 2031
- China
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- India
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- Australia
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- Rest of Asia Pacific
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- LAMEA
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Market size and forecast, by country, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Latin America
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- Middle East
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
- Africa
- Market size and forecast, by Type of Treatment, 2022-2031
- Market size and forecast, by Disease Stage, 2022-2031
- Market size and forecast, by End-User , 2022-2031
- Comparative market share analysis, 2022 & 2031
-
- Company profiles
- Otsuka Pharmaceutical Co., Ltd.
- Business overview
- Financial performance
- Type of Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- F. Hoffmann-La Roche AG
- Business overview
- Financial performance
- Type of Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Sanofi S.A.
- Business overview
- Financial performance
- Type of Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Pfizer Inc.
- Business overview
- Financial performance
- Type of Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Novartis AG
- Business overview
- Financial performance
- Type of Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Merck & Co., Inc.
- Business overview
- Financial performance
- Type of Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- AstraZeneca PLC
- Business overview
- Financial performance
- Type of Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Takeda Pharmaceutical Company Limited
- Business overview
- Financial performance
- Type of Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Teva Pharmaceutical Industries Ltd.
- Business overview
- Financial performance
- Type of Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Genzyme Corporation
- Business overview
- Financial performance
- Type of Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Otsuka Pharmaceutical Co., Ltd.
Segmentation
Key Segments Covered
Type of Treatment
- Pharmacological treatment
- Vasopressin V2 receptor antagonist
- Angiotensin converting enzyme inhibitors
- Angiotensin receptor blockers
- mTOR inhibitors
- Somatostatin analogs
- Surgical treatment
End-user
- Hospitals
- Clinics
- Ambulatory Surgical Centres
Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Rest of Europe
- Asia-Pacific
- China
- Japan
- South Korea
- India
- Australia
- Rest of Asia-Pacific
- LAMEA
- Latin America
- Middle East
- Africa
Methodology
Get your pre and post sales queries resolved by our Subject matter experts.
We will assist you to customize the report to fit your research needs.
Our prime focus is to provide qualitative and accurate data.
Feel free to order a sample report before purchase.
Your personal and confidential information is safe and secured.
© 2025 Cognate Lifesciences. All Rights Reserved.
