- Home
- Neuromyelitis Optica Spectrum Disorder Nmosd Market

Neuromyelitis Optica Spectrum Disorder (NMOSD) Market Research Report 2023
- Published Date: May, 2023 | Report ID: CLS-1936 | No of pages: 250 | Format:

Neuromyelitis Optica Spectrum Disorder (NMOSD) Market by Treatment (Monoclonal Antibody Drugs, Immunosuppressive Agents, Plasma Exchange Therapy and Other Treatments), by Route of Administration (Oral and Parenteral), by Distribution Channel (Hospitals and Clinics, Retail Pharmacies and Online Pharmacies): Global Opportunity Analysis and Industry Forecast, 2022-2031
The Neuromyelitis Optica Spectrum Disorder (NMOSD) market was valued at $420.5 million in 2022. It is projected to grow at a CAGR of 6.1% from 2023 to 2031 and reach more than $650.0 million by the end of 2031.
Analysts’ Viewpoint by Cognate Lifesciences
Neuromyelitis Optica Spectrum Disorder (NMOSD) has gained increased attention from analysts in recent years due to advancements in diagnosis, treatment, and understanding of its pathophysiology. Analysts recognize NMOSD as a rare autoimmune disorder that primarily affects the optic nerves and spinal cord, causing severe disability and impacting patients' quality of life.
Analysts have observed a shift in the treatment landscape for NMOSD, with the emergence of targeted therapies such as monoclonal antibodies. These therapies, such as Eculizumab, Inebilizumab, and Satralizumab, have shown promising results in clinical trials, effectively reducing relapse rates and improving patient outcomes. Analysts closely monitor the development, market penetration, and commercial potential of these monoclonal antibody drugs.
Furthermore, analysts emphasize the importance of immunosuppressive agents in NMOSD management. Drugs like Azathioprine, Mycophenolate Mofetil, Methotrexate, and Rituximab have demonstrated efficacy in reducing relapse rates and preserving neurological function in patients. The role of plasma exchange therapy as a treatment option for NMOSD, particularly during acute attacks or in cases of refractory disease. They monitor the market for plasma exchange equipment and services, evaluating trends in utilization and accessibility.
Neuromyelitis Optica Spectrum Disorder (NMOSD) Overview
Neuromyelitis Optica Spectrum Disorder (NMOSD) is a rare autoimmune disorder that primarily affects the central nervous system (CNS), specifically the optic nerves and spinal cord. It is often characterized by recurrent attacks of optic neuritis (inflammation of the optic nerves) and myelitis (inflammation of the spinal cord). NMOSD is distinct from multiple sclerosis (MS) and is associated with the presence of antibodies against the aquaporin-4 (AQP4) water channel. The exact cause of NMOSD is not fully understood, but it is believed to involve an autoimmune response in which the immune system mistakenly targets and damages the AQP4 channels, leading to inflammation and subsequent damage to the optic nerves and spinal cord.
Growing awareness among healthcare professionals about Neuromyelitis Optica Spectrum Disorder (NMOSD) has been a significant driver in the market. As awareness about the disorder increases, healthcare professionals become more knowledgeable about its clinical presentation, diagnostic criteria, and appropriate management strategies. This improved understanding leads to better detection and diagnosis of NMOSD cases, allowing patients to receive timely and accurate treatment. Early identification of NMOSD cases also helps prevent misdiagnosis and mistreatment, resulting in improved patient outcomes. The demand for appropriate treatment options rises with increased awareness, contributing to market growth.
The development of targeted therapies has revolutionized the treatment landscape for NMOSD. Monoclonal antibodies, such as Eculizumab, Inebilizumab, and Satralizumab, have emerged as effective treatment options. These therapies specifically target the underlying mechanisms of NMOSD, such as the inhibition of the complement system or the suppression of B-cell activity. Clinical trials have demonstrated their efficacy in reducing relapse rates, preventing disability progression, and improving quality of life for NMOSD patients. The availability of these targeted therapies addresses an unmet need in NMOSD treatment, leading to increased demand and market growth.
The expanding research and development activities in the field of NMOSD contribute significantly to market growth. Researchers are dedicated to understanding the pathophysiology of the disorder, identifying novel therapeutic targets, and exploring innovative treatment approaches. Ongoing clinical trials evaluate the safety and efficacy of new drugs and treatment regimens, providing valuable data for regulatory approvals and future treatment options. The prospect of novel therapies and the excitement surrounding research advancements attract investments and foster innovation in the field. This research-driven environment fuels market growth by expanding the treatment options available for NMOSD patients.
New product launches to flourish in the market
The Neuromyelitis Optica Spectrum Disorder (NMOSD) market is expected to see several new product launches in the coming years, driven by increasing popularity, especially as more patients seek out the convenience and cost savings offered by these facilities. Some of the key product launches expected in the Neuromyelitis Optica Spectrum Disorder (NMOSD) market are:
- In August 2020, the U.S. Food and Drug Administration (FDA) had approved Enspryng (satralizumab-mwge) for treating neuromyelitis optica spectrum disorder (NMOSD) in adults. Enspryng was given fast track designation, which expedites the development and review of drugs that are intended to treat a serious condition and show promise of meeting an unmet medical need. The drug was also designated as an orphan drug, which provides financial incentives to help and stimulate drug development for rare disorders.
- The U.S. FDA has approved Soliris (eculizumab) injection for intravenous use in the treatment of neuromyelitis optica spectrum disorder (NMOSD) in patients with anti-aquaporin-4 (AQP4) antibody positive.
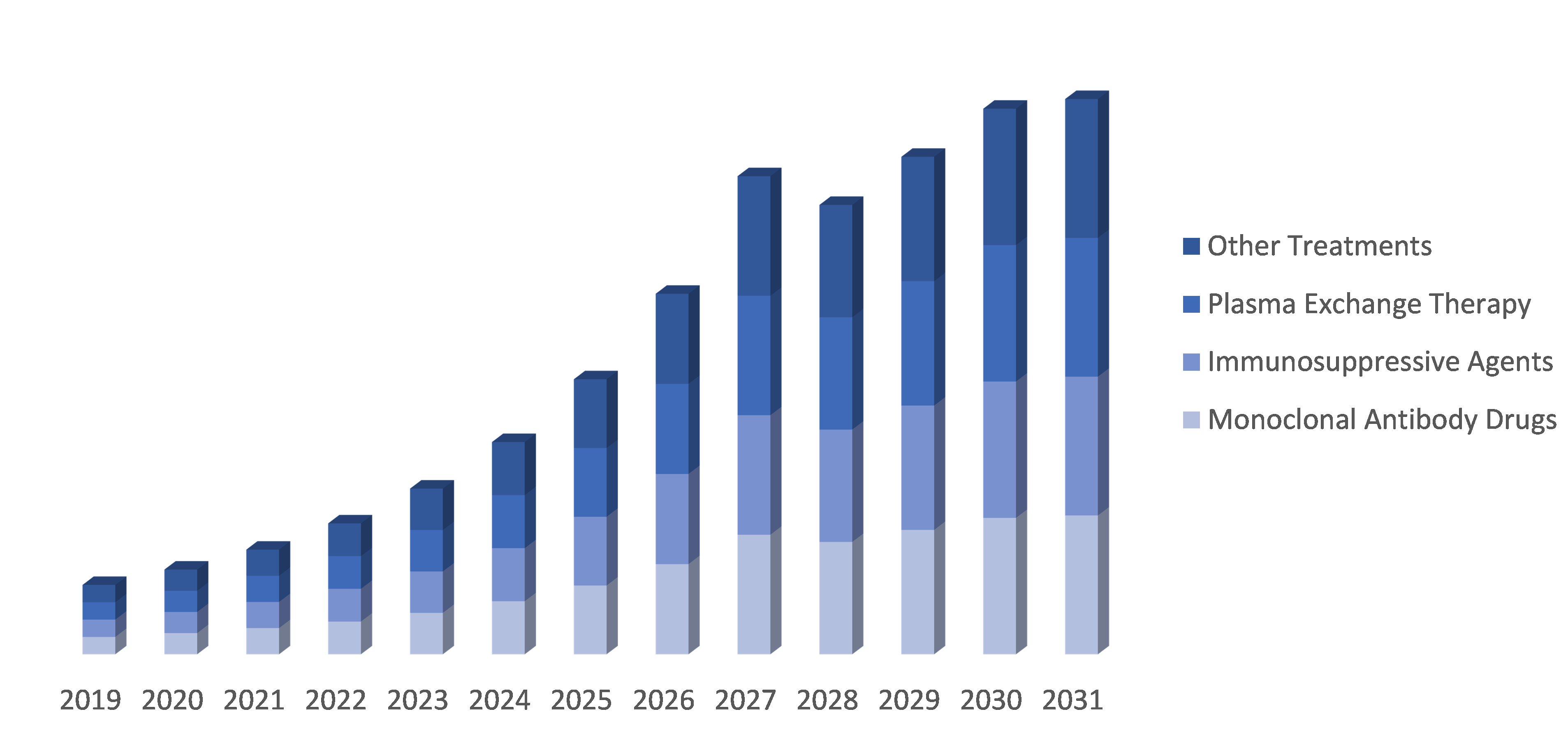
Segment Overview:
By Treatment stage: The Neuromyelitis Optica Spectrum Disorder (NMOSD) market is divided into monoclonal antibody drugs, immunosuppressive agents, plasma exchange therapy and other treatments. These different treatment options aim to address the symptoms and underlying mechanisms of NMOSD. Monoclonal antibody drugs and immunosuppressive agents target the immune system, while plasma exchange therapy helps remove harmful antibodies. Other treatments may involve symptom management or supportive care for patients with NMOSD.
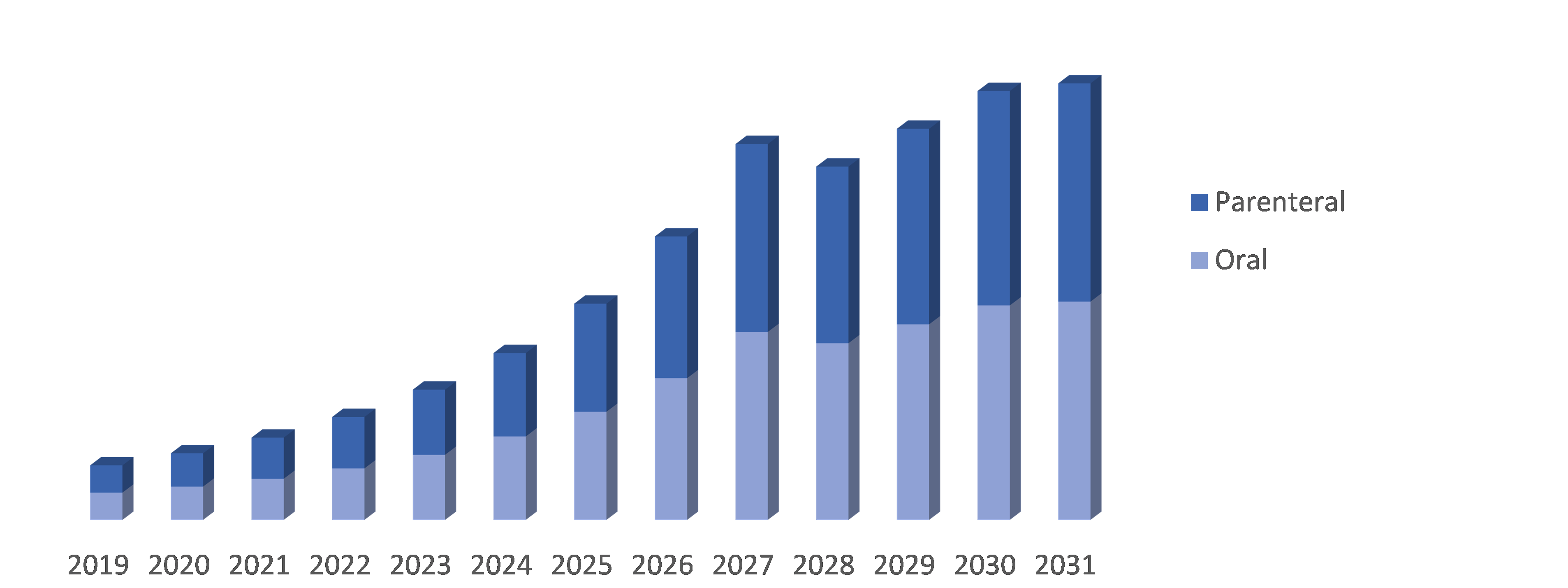
By Route of Administration: The Neuromyelitis Optica Spectrum Disorder (NMOSD) market is segmented into Oral and Parenteral. Oral administration refers to medications that are taken by mouth, such as tablets or capsules. Parenteral administration refers to medications that are administered through non-oral routes, such as injections (subcutaneous, intramuscular, or intravenous) or infusions. This segmentation provides different options for delivering treatments to patients with NMOSD based on their specific needs and preferences.
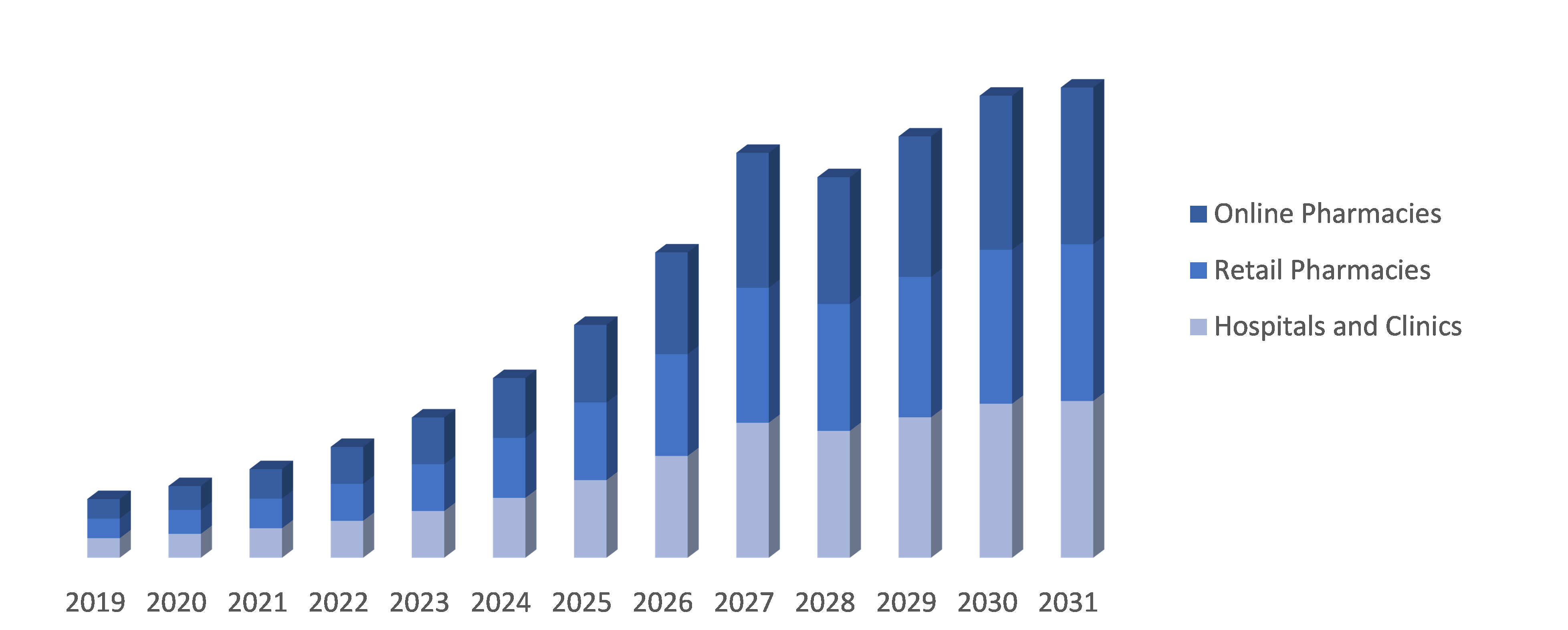
By Distribution Channel: The Neuromyelitis Optica Spectrum Disorder (NMOSD) market is segmented into Hospitals and Clinics, Retail Pharmacies and Online Pharmacies. Hospitals and clinics serve as primary distribution channels for NMOSD treatments, providing direct access to healthcare professionals. Retail pharmacies offer convenient access to medications for patients, while online pharmacies provide the option of ordering medications remotely and having them delivered to their doorstep. This segmentation ensures various avenues for patients to obtain NMOSD treatments based on their preferences and accessibility.
By Region:
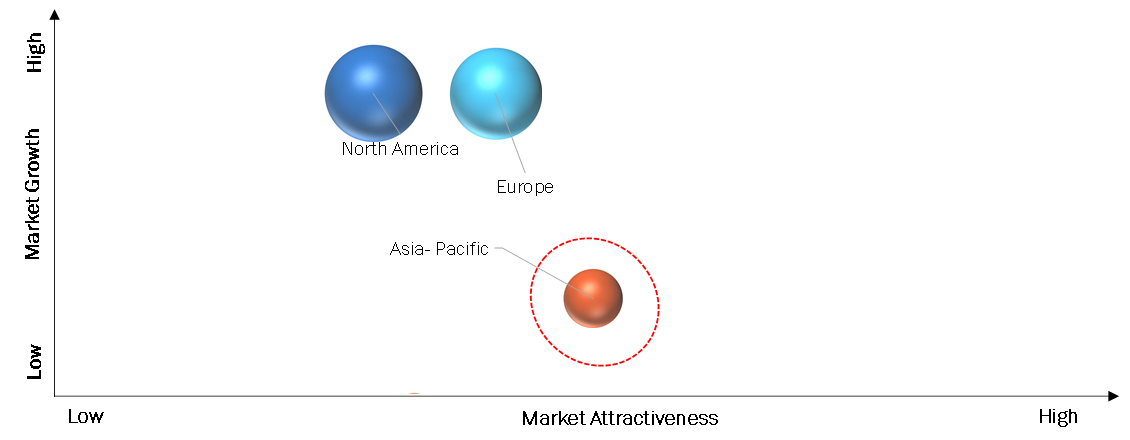
The North American Neuromyelitis Optica Spectrum Disorder (NMOSD) market is experiencing growth and is characterized by various factors. The market is driven by increasing awareness of NMOSD among healthcare professionals and patients, leading to improved diagnosis rates. The availability of advanced treatment options, such as monoclonal antibody drugs and immunosuppressive agents, contributes to market expansion. Additionally, the presence of well-established healthcare infrastructure, including hospitals, clinics, and retail pharmacies, supports efficient distribution and accessibility of NMOSD treatments. Furthermore, ongoing research and development efforts aim to enhance therapeutic outcomes and expand the treatment landscape, making North America a significant region in the NMOSD market.
The Asia Pacific (APAC) Neuromyelitis Optica Spectrum Disorder (NMOSD) market is witnessing significant growth and presents numerous opportunities. The market growth can be attributed to factors such as the rising prevalence of NMOSD in the region, increasing awareness among healthcare professionals and patients, and improving healthcare infrastructure. APAC countries are witnessing advancements in healthcare technology and the availability of innovative treatment options for NMOSD. Moreover, government initiatives aimed at improving healthcare access and affordability are further driving market growth. The presence of a large patient population in countries like China and India also contributes to the expansion of the APAC NMOSD market.
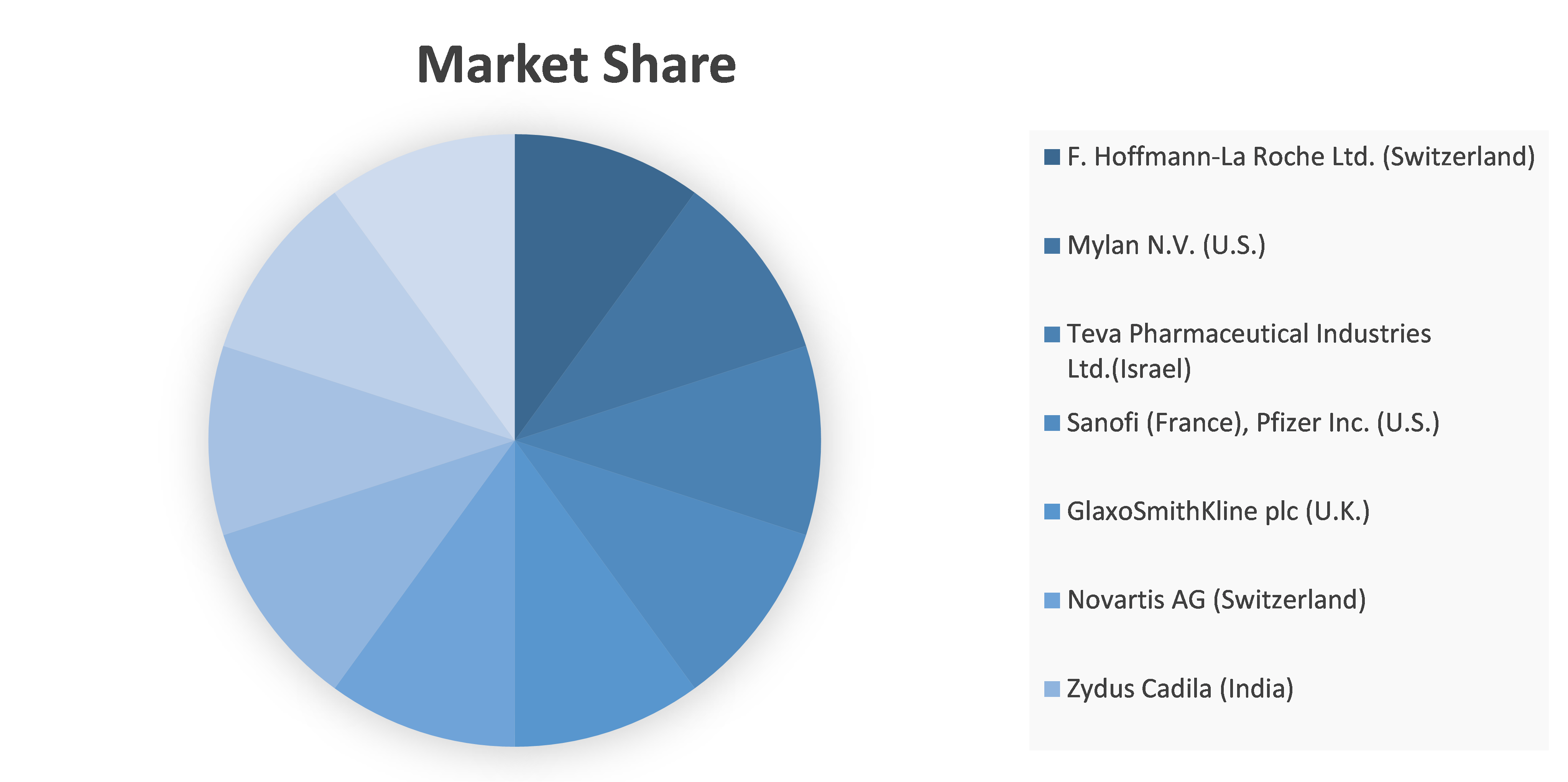
Competitive analysis and profiles of the major players in the Neuromyelitis Optica Spectrum Disorder (NMOSD) market, such as F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd., Sanofi, Pfizer Inc., GlaxoSmithKline plc, Novartis AG, Zydus Cadila, Boehringer Ingelheim International GmbH., Apotex Inc., AstraZeneca and Horizon Therapeutics PLC. Major players have adopted product launch and acquisition as key developmental strategies to improve the product portfolio of the Neuromyelitis Optica Spectrum Disorder (NMOSD) market.
Market Scope and Structure Analysis
|
Report Metric |
Details |
|
Market Size Available for Years |
2021–2031 |
|
Base Year Considered |
2022 |
|
Forecast Period |
2023–2031 |
|
Forecast Unit |
Value (USD) |
|
Segments Covered |
By Treatment Type, Route of Administration, Distribution Channel and Region |
|
Regions Covered |
North America, Europe, Asia-Pacific, LAMEA |
|
Companies Covered |
|
Key Segments Covered
Treatment
- Monoclonal Antibody Drugs
- Immunosuppressive Agents
- Plasma Exchange Therapy
- Other Treatments
Route of Administration
- Oral
- Parenteral
Distribution Channel
- Hospitals and Clinics
- Retail Pharmacies
- Online Pharmacies
Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Rest of Europe
- Asia-Pacific
- China
- Japan
- South Korea
- India
- Australia
- Rest of Asia-Pacific
- LAMEA
- Latin America
- Middle East
- Africa
TABLE OF CONTENT
- Research Methodology
- Desk Research
- Real-time insights and validation
- Forecast model
- Assumptions and forecast parameters
- Assumptions
- Forecast parameters
- Data sources
- Primary
- Secondary
- Executive Summary
- 360° summary
- By Treatment trends
- By Route of Administration trends
- By Distribution Channel
- Market Overview
- Market segmentation & definitions
- Key takeaways
- Top investment pockets
- Top winning strategies
- Porter’s five forces analysis
- Bargaining power of consumers
- Bargaining power of suppliers
- Threat of new entrants
- Threat of substitutes
- Competitive rivalry in the market
- Market dynamics
- Drivers
- Restraints
- Opportunities
- Technology landscape
- Pipeline Analysis
- Regulatory landscape
- Patent landscape
- Market value chain analysis
- Strategic overview
- Neuromyelitis Optica Spectrum Disorder (NMOSD) Market, by Treatment
- Monoclonal Antibody Drugs
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Immunosuppressive Agents
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Plasma Exchange Therapy
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Other Treatments
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Monoclonal Antibody Drugs
- Neuromyelitis Optica Spectrum Disorder (NMOSD) Market, by Route of Administration
- Oral
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Parenteral
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Oral
- Neuromyelitis Optica Spectrum Disorder (NMOSD) Market, by Distribution Channel
- Hospitals and Clinics
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Retail Pharmacies
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Online Pharmacies
- Market size and forecast, by region, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Hospitals and Clinics
- Neuromyelitis Optica Spectrum Disorder (NMOSD) Market, by Region
- North America
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Market size and forecast, by country, 2022-2031
- Comparative market share analysis, 2022 & 2031
- U.S.
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Canada
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Mexico
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Europe
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Market size and forecast, by country, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Germany
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- UK
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- France
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Spain
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Italy
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Rest of Europe
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Asia Pacific
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Market size and forecast, by country, 2022-2031
- Comparative market share analysis, 2022 & 2031
- China
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- India
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Australia
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Rest of Asia Pacific
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- LAMEA
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Market size and forecast, by country, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Latin America
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Middle East
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- Africa
- Market size and forecast, by Treatment, 2022-2031
- Market size and forecast, by Route of Administration, 2022-2031
- Market size and forecast, by Distribution Channel, 2022-2031
- Comparative market share analysis, 2022 & 2031
- North America
- Company profiles
- F. Hoffmann-La Roche Ltd
- Business overview
- Financial performance
- Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Mylan N.V
- Business overview
- Financial performance
- Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Teva Pharmaceutical Industries Ltd.
- Business overview
- Financial performance
- Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Sanofi
- Business overview
- Financial performance
- Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Pfizer Inc.
- Business overview
- Financial performance
- Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- GlaxoSmithKline plc
- Business overview
- Financial performance
- Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Novartis AG
- Business overview
- Financial performance
- Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Zydus Cadila
- Business overview
- Financial performance
- Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Boehringer Ingelheim International GmbH
- Business overview
- Financial performance
- Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- Apotex Inc.
- Business overview
- Financial performance
- Treatment portfolio
- Recent strategic moves & developments
- SWOT analysis
- F. Hoffmann-La Roche Ltd
Segmentation
Key Segments Covered
Treatment
- Monoclonal Antibody Drugs
- Immunosuppressive Agents
- Plasma Exchange Therapy
- Other Treatments
Route of Administration
- Oral
- Parenteral
Distribution Channel
- Hospitals and Clinics
- Retail Pharmacies
- Online Pharmacies
Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Rest of Europe
- Asia-Pacific
- China
- Japan
- South Korea
- India
- Australia
- Rest of Asia-Pacific
- LAMEA
- Latin America
- Middle East
- Africa
Methodology
Get your pre and post sales queries resolved by our Subject matter experts.
We will assist you to customize the report to fit your research needs.
Our prime focus is to provide qualitative and accurate data.
Feel free to order a sample report before purchase.
Your personal and confidential information is safe and secured.
© 2025 Cognate Lifesciences. All Rights Reserved.
